How Do Balanced Chemical Equations Show the Conservation of Mass
We create a. How do you know if a chemical equation is balanced.
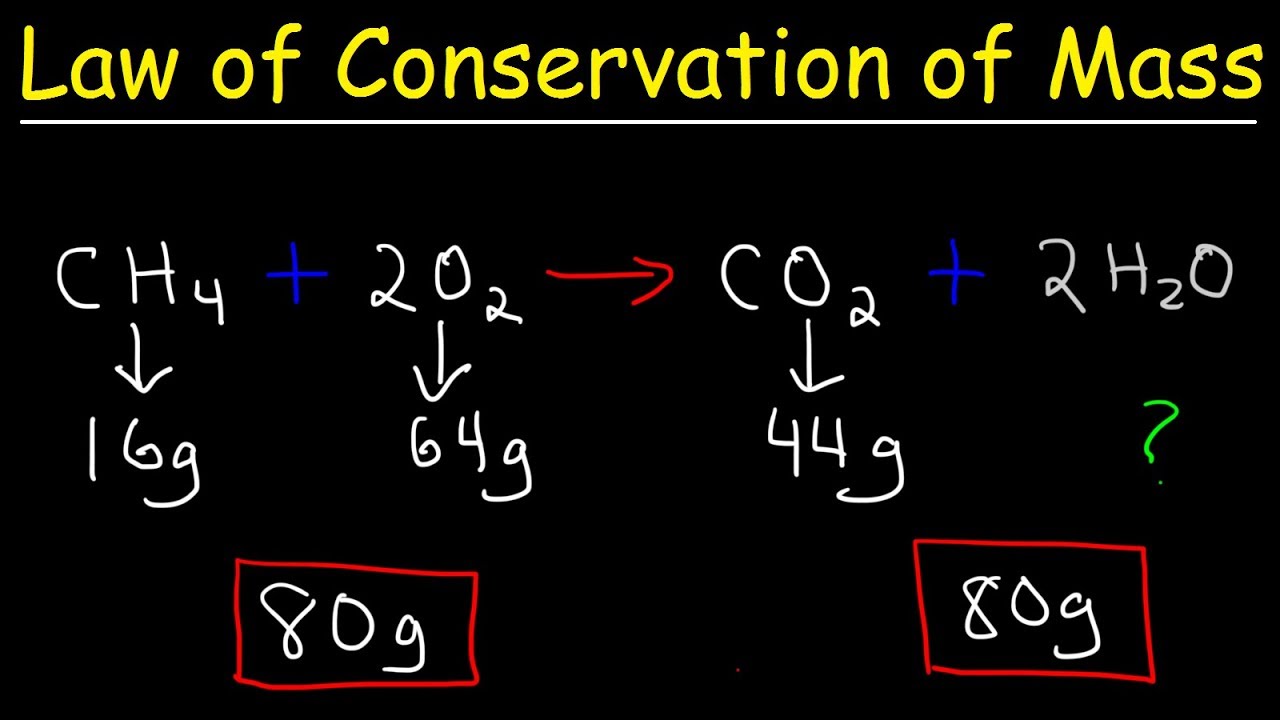
Law Of Conservation Of Mass Fundamental Chemical Laws Chemistry Youtube
Products are listed on the right side of the equation.
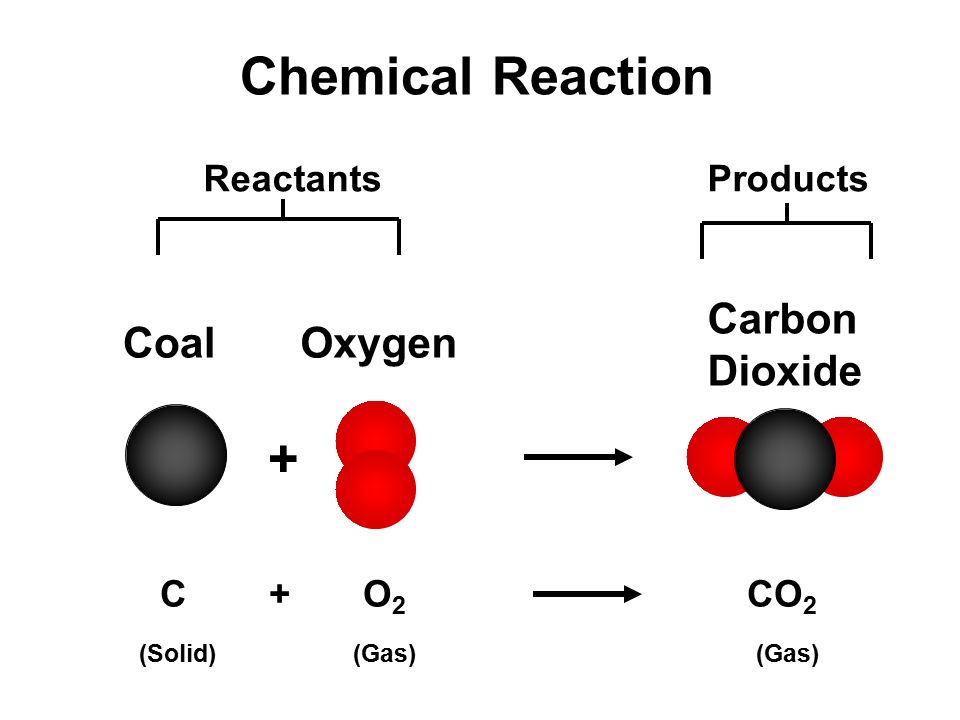
. Write the unbalanced equation. This figure illustrates graphically what the numbers above show. Rusting of iron the setting of milk into curd digestion of food.
Thus the task involves using the above equations the given information and your understanding of Newtons laws to determine the acceleration. An equation is balanced in order to satisfy the law of conservation of mass according to which total mass of the reactants is equal to the total mass of the products ie mass can neither be created nor be destroyed during any chemical change. Chemical Reactions and Equations.
The function crosses zero at approximately x 15. Construct an explanation based on evidence to describe conservation of matter in a chemical reaction including the resulting differences between products and reactants. Chemical formulas of reactants are listed on the left side of the equation.
Explain the role of the Law of Conservation of Mass in a chemical reaction. They show the physical state of that substance. During electrolysis of water the gas collected in one test tube is double than the other why.
Basically this means there are the same numbers of each type of atoms on the left side of the equation as. The same atoms that were. The balanced equation for the reaction of interest contains the stoichiometric ratios of the reactants and products.
To get a more precise value we must actually solve the function numerically. Lets consider a chemical equation this time. Balanced and unbalanced chemical equations and balancing of chemical equations.
There are essentially three steps to the process. If mass m and net force F net are known then the acceleration is determined by use of the equation. A chemical equation describes what happens in a chemical reactionThe equation identifies the reactants starting materials and products resulting substances the formulas of the participants the phases of the participants solid liquid gas the direction of the chemical reaction and the amount of each substance.
False - Mass is independent of the gravitational environment that an object is in and dependent solely upon the number of atoms in the object and the type of atoms Carbon. 10262021 Create an account. This means that chemical reactions can be represented by.
In chemical reactions atoms are never created or destroyed. Na 2 Cl 2 NaClunbalanced equation The example has two atoms of sodium and two atoms of chlorine at LHS. Balanced chemical equations sometimes include state symbols in brackets after each formula.
Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products. To gain a feel for how this method is applied try the. Reactants and products are separated by putting an arrow between them to show the direction of the reaction.
Learn more about balanced chemical equations understand the law of conservation of mass and practice the steps involved to balance several example equations. Obtain evaluate and communicate information about the law of conservation of energy to develop. The law of conservation of mass dictates that the quantity of an element does not change over the course of a reaction.
ANTOINE LAVOISER INTRODUCED LAW OF CONSERVATION OF MASS 4. It takes practice to be able to write balanced equations. If you recall formulae of magnesium oxygen and magnesium oxide the above word-equation can be written as MgO2 MgO 5.
These ratios can be used as. A balanced chemical equation tells you the amounts of reactants and products needed to satisfy the Law of Conservation of Mass. The transformation of chemical substance into another chemical substance is known as Chemical Reaction.
Balance chemical equations calculator highlights the user if the equation is unbalanced. Chemical equations are balanced for mass and. What is a chemical reaction Class 10.
What can you change to balance an equation. A F net m Your Turn to Practice. Because of this mass is.
We use the function funcscipyoptimizefsolve to do that. The mass of an object is mathematically related to the weight of the object. Evidence could include models such as balanced chemical equations S8P2.
More precisely we want to solve the equation fx cosx 0. Therefore a chemical equation is balanced when all elements have equal values on both the left and right sides. A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products and shows the direction of the reaction.
Writing a chemical reaction A chemical equation represents a chemical reaction. Balancing a chemical reaction To balance a chemical equation first draw boxes. Atoms of sodium and one atom.
Play a game to test your ideas. As per the law of mass conservation it is not possible so the given equation is unbalanced.

Balanced Chemical Equations Law Of Conservation Of Matter Mass Law Of Constant Ratios Science Online

Law Of Conservation Of Mass How To Balance Equations And Solve Problems Pp I Part I Youtube

Balancing Chemical Equations And The Law Of Conservation Of Mass Conservation Of Mass Chemical Equation Equations
No comments for "How Do Balanced Chemical Equations Show the Conservation of Mass"
Post a Comment